Source: Foxtechview.com
Introduction:
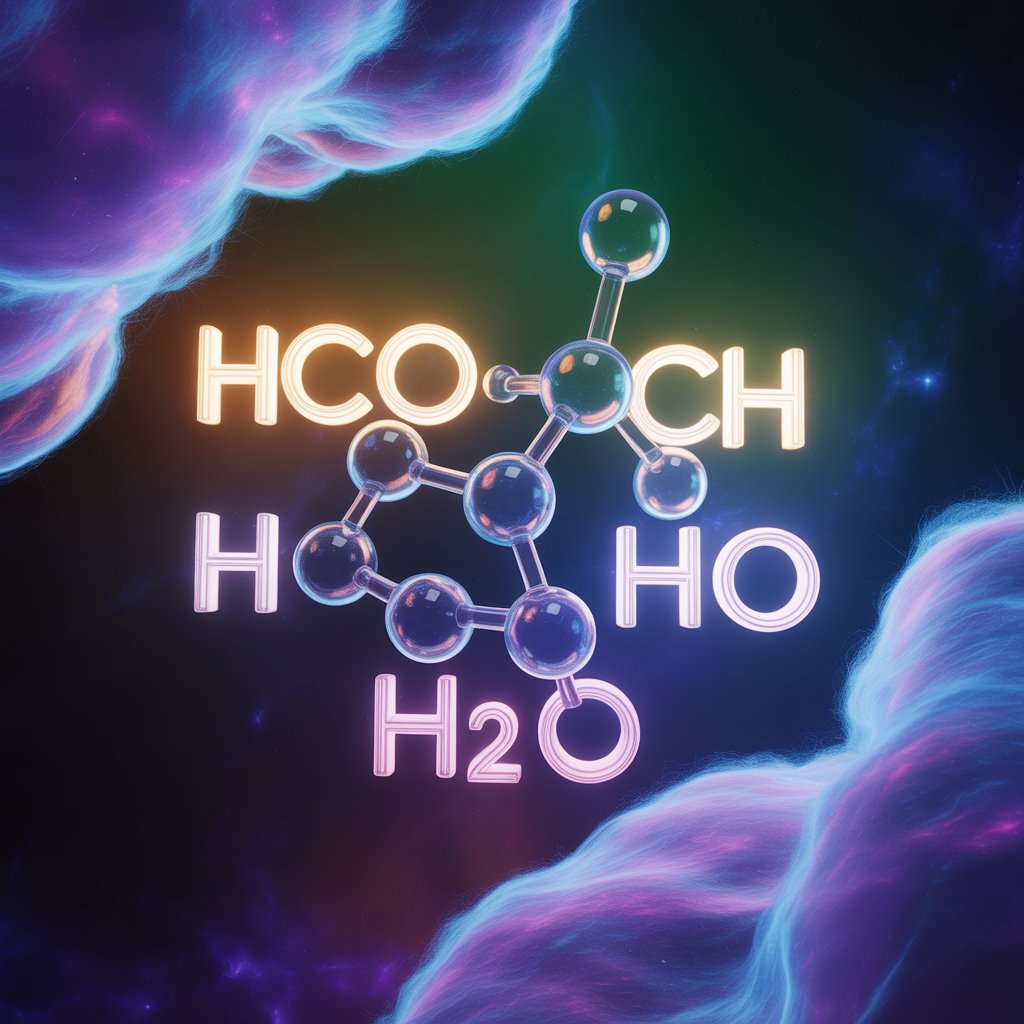
The keyword hcooch ch2 h2o represents a system of molecules that includes formate (HCOO), water (H2O), and a methylene (CH2) group. These molecular compounds are interesting in organic chemistry because they can form a hydrogen-bonded networks and have distinct structural and stability properties. This article examines the possible structure of hcooch ch2 h2o. It highlights the bonding interactions and discusses thermodynamic stability using theoretical chemistry.
The following is a brief introduction to the topic:
The structural complexity and reactivity of organic molecules with ester and hydrate functionalities are often studied. The notation of hcooch ch2 h2o indicates the presence of a hydrated ester-like system that is possibly related to methyl formate or a derivative compound. The shorthand does not directly correspond to a standard IUPAC formulation, but it can be used as a tool for analyzing structure, intermolecular interaction, and stability trends.
It is important to understand these systems because they are present in industrial processes, atmospheric chemistry, and solvent interactions. These molecules are often used as precursors for alcohols, acids, and intermediates in hydrolysis. They can also be used as a model to study hydrogen bonds in mixed organic and aqueous phases.
Molecular Structure Considerations
The molecular form hcooch ch2 h2o is a derivative of formyl ester, or formate, associated with a fragment of methylene and a water molecule. This implies two domains:
- The Ester or Formate Moiety
- The carbonyl group is polar and can accept hydrogen bonds.
- In hydrogen-bonded interactions, the adjacent oxygen atom can act as a site of donation.
- The CH2 Group
- It contains a non-polar, hydrophobic segment that influences solubility and packing between molecules.
- It can be substituted or stabilized depending on the bonding environment.
- The Hydrate Component H2O
- Both hydrogen bond donor as well as acceptor.
- Stabilizes system by intermolecular association. Hydrogen-bonding motif can be cyclic.
The overall geometry is most likely to be a cluster of hydrogen-bonded molecules, in which the water molecule coordinates with the ester oxygen. This arrangement decreases internal strain, and improves the stability of the system when in polar environments.
Bonding Interactions
The stability of the hcooch ch2 h2o system is governed primarily by noncovalent interactions.
- Hydrogen Bonding:
- The hydrogen atoms in H2O are strongly interacted with by the carbonyl oxygen.
- This interaction slows down the potential hydrolysis by reducing the reactivity.
- Dipole-Dipole Interactions:
- The alignment of the polar groups carbonyl (C) and hydroxyl (OH) minimizes free energy.
- This interaction dominates in aqueous solution, which promotes solubility.
- Van der Waals Forces:
- The CH2 segment is weakly dispersive.
- These forces are secondary, but they play a part in the molecular packing as well as phase behavior.
Stability Analysis
The environmental conditions, namely solvent, temperature and pressure, affect the stability of hcooch ch2 h2o.
- The Gas Phase
- The hydrogen bonding is weaker in the absence of bulk solvent.
- Clusters can still be intact, but are less stable than solutions.
- In Solution (Aqueous Media or Polar Media).
- Water molecules reinforce hydrogen bonding networks.
- It is often a dimer or extended cluster that forms when the hydrate becomes highly stabilized.
- Thermodynamics
- Strong hydrogen bonds are responsible for enthalpic stabilization.
- Entropy contributions are dependent on the degree of molecular association. Excessive clustering can reduce entropy and balance overall stability.
- Kinetic Aspects
- The ester moiety can be hydrolyzed to form formic acid or methanol derivatives.
- Water is a catalyst that can be used to facilitate hydrolysis and stabilize the parent molecule.
Experimental and Computational Approaches
Researchers use a combination spectroscopic tools and computational tools to analyze hcooch ch2 h2o:
- Infrared (IR), and Raman Spectroscopy
- The hydrogen bond can be confirmed by detecting the vibrational modes and stretching of O-H group as well as cthe arbonyl groups.
- Nuclear Magnetic Resonance
- This tool provides information on the chemical environment surrounding protons and carbons in particular the CH2 region and the hydroxyl region.
- Quantum Chemical Calculations
- Density Functional Theory helps predict binding energies and optimize structures.
- Simulations of molecular dynamics reveal dynamic behavior of hydrate clusters in solution.
The combination of these methods provides insight into the relative stability and structural characteristics of the compound.
Applications and Relevance
The structural motif of hcooch ch2 h2o can be applied to several different domains.
- Atmospheric chemistry: Hydrated clusters of organic compounds play a key role in the formation of secondary organic aerosols and cloud condensation.
- Industrial Chemistry: Understanding ester hydrates is important for polymer chemistry and reaction engineering.
- Hydrated small molecules are often used as models to study biomolecular water interactions, especially in the case of protein-water interactions.
Conclusion
The molecular structure hcooch ch2 h2o shows how water can act as a hydrogen bond to stabilize organic molecules. The structure of the cluster is composed of a methylene fragment and a polar ester group. Together, they form a stable cluster in aqueous conditions. Analyzing such systems helps us to better understand molecular interactions and thermodynamic stability. It also allows us to better understand the role that hydration plays in chemical processes.
Future research may include improving computational models, performing spectroscopic tests, and exploring analogs in industrial and environmental settings.
Read more informative articles; visit this website:Fox Tech View
FAQs about hcooch ch2 h2o and Reactions
1. What happens if formic acid is mixed with water?
Partial ionization occurs when formic acid (HCOOH), which dissolves in water.
HCOOH +H2O = H3O++HCOOHCOOH
This equilibrium shows formic acid to be a weak pH. It donates a proton into water to produce hydronium and formate ions.
2. What is the result of CH3-CH=CH2 when H2O is present?
Propene is an alkene. It undergoes Hydration Reaction in the presence of an acid catalyst to form Propan-2-ol.
CH3-CH=CH2 + H2O – CH3-CHOH-CH3CH3-CH=CH2 \; + \; H2O \; – \; CH3-CHOH-CH3CH3 -CH=CH2 +H2 O-CH3 -CHOH-CH3
The -OH group is attached to the carbon with the most substituted carbon.
3. What is the type of reaction CH2 + O2 CO2 + H2O produces?
This is a combustion. The methylene (CH2) group reacts with oxygen to form carbon dioxide (CO2) as well as water. This reaction releases heat and light. Combustion is a highly exothermic reaction that is fundamental to energy production.
4. What is the interaction of HCOOH with water?
The carbonyl oxygen molecules (C=O), when formic acid is dissolved in water, form strong hydrogen bonds. These interactions stabilize the solutions and promote partial Ionization leading to an acidic environment.
5. What reaction occurs when hcooch ch2 is mixed with h2o
The keyword hcoochch2h2o is a ester-like hydration that involves a formate fragment, a water, and a methylene group. Its reactions pathways include:
-
Hydrolysis produces formic acid HCOOH and other alcohol derivatives.
-
Formation of hydrogen-bond stabilizing complexes in aqueous medium.
-
Decomposition by heat yielding CO2and smaller organics
6. What is the use of hcooch Ch2 H2o?
hcoochch2h2o is a chemical that has important structures in:
-
Atmospheric Chemistry
-
Modeling the hydration of organic compounds
-
Research Applications for studying ester hydrolysis.
7. What is the relationship between HCOOH, CH2H2O, and formic acid?
This compound is a unit (HCOO). which is directly derived by formic acid (HCOOH). It mimics formic acid’s interactions with water in hydrated form. This provides insights into the acidity and solubility of formic acids.
8. What’s the formula for HCOOCH₂CH₂O?
The HOOCH CH₂ H₂O representation is a shorthand and not a strict IUPAC molecular formula. It implies a combination:
-
A formate ester unit (HCOOCH),
-
A methylene group (CH₂),
-
One water molecule is H₂O.
It is better to describe it as an organic complex than a well-defined single molecule.
9. What chemical formula is hcooch Ch2 H2o?
The shorthand HCOOCH₂H₂Ois not a chemical compound. However, the expanded meaning could be written as:
C₂H₆O₃ (approximate empiricalrepresentation) )C_2H6O3 \quad \text(approximate empirical representation)C2 H6 O3 (approximate empirical representation)
This indicates the presence of carbon, oxygen, and hydrogen atoms.